Building Supply Chain Resilience: Why Nearshoring Matters for Medical Device Manufacturers
- TN Plastics

- Dec 12, 2025
- 6 min read
The COVID-19 pandemic, geopolitical disruptions, and recent global supply chain challenges have exposed critical vulnerabilities in medical device manufacturing. For device manufacturers relying on distant offshore suppliers, these disruptions have translated into production delays, quality inconsistencies, and compromised patient care. As the medical device industry rethinks supply chain strategies, nearshoring has emerged as a powerful solution for building resilience, reducing risk, and maintaining competitive advantage. At TN-plastics, our strategic manufacturing footprint in Michigan and Italy positions us as the nearshoring partner medical device OEMs need to navigate an increasingly uncertain global landscape.
The Supply Chain Crisis: Lessons Learned
Recent years have delivered hard lessons about supply chain fragility. Medical device manufacturers have experienced unprecedented disruptions affecting every aspect of component sourcing and production.
Key Supply Chain Vulnerabilities Exposed
Extended Lead Times: Ocean freight disruptions and port congestion have extended component delivery times from weeks to months, forcing manufacturers to maintain excessive safety stock or face production delays.
Quality and Compliance Risks: Distance from overseas suppliers makes quality oversight challenging. FDA warning letters and import suspensions have affected multiple foreign component suppliers, disrupting medical device production.
Single-Source Dependency: Over-reliance on suppliers in specific geographic regions creates concentration risk. When a single region experiences disruption, manufacturers lack backup options.
Communication and Collaboration Challenges: Time zone differences, language barriers, and cultural disconnects impede effective problem-solving and design collaboration, extending development timelines.
Regulatory Complexity: Managing FDA and international regulatory requirements across distant supply chains adds complexity, documentation burden, and compliance risk.
These vulnerabilities have prompted medical device manufacturers to fundamentally rethink supply chain strategies, with nearshoring emerging as a strategic priority for forward-thinking organizations.
Understanding Nearshoring: More Than Geographic Proximity
Nearshoring involves partnering with manufacturing suppliers located closer to your operations—in the same country or region—rather than relying on distant offshore facilities. For medical device manufacturers, nearshoring offers advantages that extend far beyond geography.
Strategic Benefits of Nearshoring
Reduced Lead Times: Proximity enables faster component delivery, reducing inventory carrying costs and improving responsiveness to demand changes. TN-plastics facilities in Michigan and Italy serve North American and European markets with significantly shorter lead times than offshore alternatives.
Enhanced Quality Oversight: Geographic proximity facilitates regular site visits, real-time problem-solving, and collaborative quality improvement. Face-to-face engagement builds stronger partnerships and faster resolution of issues.
Regulatory Familiarity: Nearshore partners understand FDA and regional regulatory requirements, speak the same regulatory language, and maintain documentation systems aligned with local expectations. TN-plastics FDA registration (3014717638) and ISO 13485:2016 certification demonstrate this regulatory alignment.
Time Zone and Communication Advantages: Operating in similar time zones enables real-time communication, faster decision-making, and more effective collaboration throughout product development and production.
IP Protection: Manufacturing within regions with strong intellectual property protections reduces risks of design theft or unauthorized reproduction of proprietary components.

The Business Case for Nearshoring Medical Device Components
While nearshoring may carry higher upfront labor costs than offshore alternatives, a comprehensive total cost of ownership analysis reveals compelling financial advantages.
Total Cost of Ownership Considerations
Reduced Transportation Costs: Lower freight expenses and shorter shipping distances
Inventory Optimization: Reduced safety stock requirements due to shorter, more predictable lead times
Lower Carrying Costs: Decreased working capital tied up in in-transit and buffer inventory
Minimized Disruption Costs: Fewer production delays and emergency expedite fees
Improved Cash Flow: Faster inventory turns and more efficient working capital management
Quality Cost Reduction: Fewer defects, returns, and warranty claims through better oversight
Competitive Advantages Beyond Cost
Nearshoring delivers strategic benefits that strengthen competitive positioning:
Faster Time-to-Market: Accelerated product development through improved collaboration
Market Responsiveness: Ability to quickly adjust to demand changes or design modifications
Innovation Enablement: Closer collaboration facilitates design optimization and value engineering
Supply Chain Transparency: Better visibility into supplier operations and performance
Sustainability Benefits: Reduced carbon footprint from shorter transportation distances
TN-plastics: Purpose-Built for Nearshoring Success
TN-plastics strategic manufacturing footprint and comprehensive capabilities position us as the ideal nearshoring partner for medical device OEMs seeking supply chain resilience.
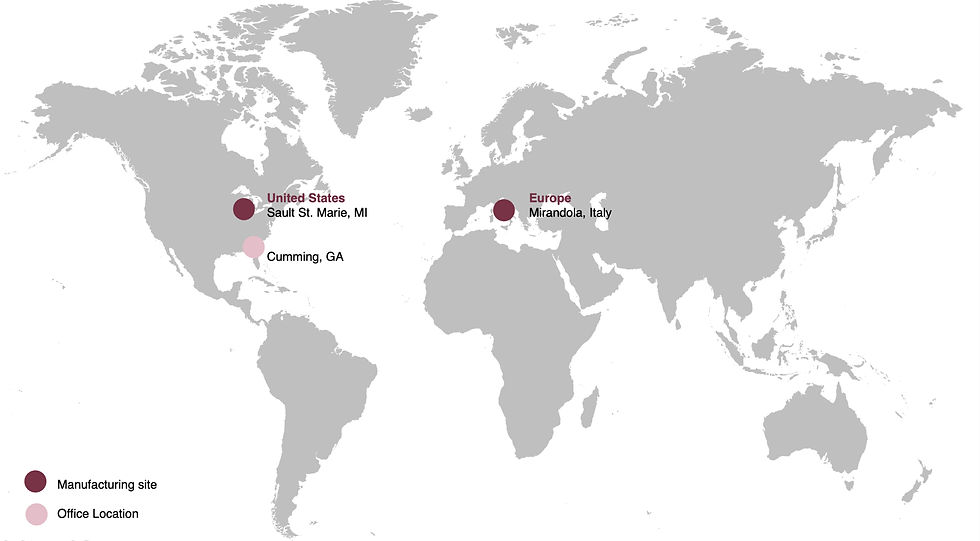
Strategic Geographic Presence
Michigan Facility (Sault Ste. Marie): Serving North American medical device manufacturers with FDA-registered, Class 8 compliant cleanroom manufacturing. Founded in 1913, our Michigan facility brings over a century of precision manufacturing expertise to medical device component production.
Italy Facility (Mirandola): Supporting European medical device manufacturers in the heart of Italy medical device cluster. Proximity to major European OEMs enables responsive service and regulatory alignment with MDR requirements.
Global Tsubaki Nakashima Network: Access to 20 plants across 10 countries provides supply chain redundancy and scalability while maintaining regional manufacturing capabilities.
Comprehensive Manufacturing Capabilities
Contract injection molding with capacity from 1-50 million pieces annually
Precision plastic ball manufacturing up to 500 million pieces annually
Class 8 compliant cleanroom manufacturing for contamination-sensitive applications
In-house mold design and building capabilities reducing tooling lead times
Complete validation services (IQ/OQ/PQ) supporting regulatory submissions
Over 80 plastic formulations including biocompatible medical-grade materials

Building Supply Chain Redundancy Through Dual-Source Strategies
Smart medical device manufacturers are moving beyond nearshoring to implement dual-source strategies that provide additional resilience through qualified backup suppliers.
Benefits of Dual-Source Manufacturing
Continuity Assurance: If one facility experiences disruption, production continues at the second source without interruption.
Capacity Flexibility: Multiple sources enable rapid scaling to meet demand surges or support new product launches.
Competitive Leverage: Qualified alternatives provide negotiating leverage while maintaining supplier relationships.
Geographic Risk Mitigation: Regional diversity protects against localized disruptions from natural disasters, labor issues, or political instability.
TN-plastics Dual-Source Advantages
Our Michigan and Italy facilities provide natural dual-source capabilities for medical device manufacturers:
Standardized quality systems (ISO 13485:2016) across locations ensuring consistent output
Unified project management and engineering support coordinating between facilities
Tool transfer capabilities enabling production flexibility between locations
Shared material qualification and supplier relationships
Consistent validation and documentation standards supporting regulatory requirements
Implementing a Successful Nearshoring Strategy
Transitioning to nearshore manufacturing partners requires thoughtful planning and execution to ensure smooth implementation and realize anticipated benefits.
Key Implementation Steps
1. Conduct Total Cost Analysis: Evaluate all costs including freight, inventory carrying costs, quality costs, and disruption risks. Compare total cost of ownership rather than piece price alone.
2. Assess Component Priorities: Identify critical components where supply reliability, quality oversight, or time-to-market justify nearshoring investment. Start with highest-risk or highest-value components.
3. Evaluate Partner Capabilities: Assess potential nearshore partners on technical capabilities, quality systems, regulatory compliance, and cultural fit. Site visits and capability audits are essential.
4. Plan Tool Transfer: Work with your nearshore partner on tool evaluation, transfer logistics, and validation runs to ensure seamless transition. TN-plastics specialized mold transfer services minimize disruption.
5. Implement Parallel Production: Run parallel production during transition to verify quality equivalence and build confidence before full transition.
6. Establish Performance Metrics: Define clear KPIs for quality, delivery, responsiveness, and total cost to measure nearshoring success.
Avoiding Common Nearshoring Pitfalls
Focusing solely on piece price rather than total cost of ownership
Underestimating tool transfer complexity and validation requirements
Rushing implementation without adequate planning and parallel validation
Failing to align on quality expectations and documentation requirements upfront
Overlooking cultural fit and communication compatibility
Sustainability and Environmental Benefits of Nearshoring
Nearshoring delivers significant environmental benefits that align with growing corporate sustainability commitments and stakeholder expectations.
Reduced Carbon Footprint
Shorter transportation distances dramatically reduce freight emissions
Elimination of intercontinental ocean freight and air cargo reduces Scope 3 emissions
Reduced packaging requirements for shorter shipping distances
Lower inventory volumes reduce warehouse energy consumption
Sustainable Manufacturing Practices
TN-plastics commitment to sustainability through the Tsubaki Nakashima global network includes:
ISO 14001:2015 environmental management system certification
Energy efficiency initiatives and renewable energy investments
Waste reduction and recycling programs
Water conservation and responsible resource management
Eco-friendly plastic alternatives and sustainable material options
The Future of Medical Device Supply Chains
The trend toward nearshoring and supply chain resilience will continue shaping medical device manufacturing strategies in coming years.
Industry Trends Driving Nearshoring
Government incentives encouraging domestic and regional manufacturing
Regulatory emphasis on supply chain security and transparency
Investor and stakeholder pressure for supply chain risk management
Growing importance of ESG considerations in supply chain decisions
Rising demand for agile, responsive supply chains supporting innovation
Preparing for the Future
Forward-thinking medical device manufacturers are taking proactive steps to build resilient supply chains:
Conducting comprehensive supply chain risk assessments
Diversifying supplier base and qualifying nearshore alternatives
Investing in stronger supplier partnerships and collaboration
Implementing supply chain visibility and monitoring systems
Aligning sourcing strategies with long-term business and sustainability goals
Conclusion: Building Resilience Through Strategic Nearshoring
Supply chain resilience has moved from competitive advantage to business imperative for medical device manufacturers. Nearshoring offers a proven strategy for reducing risk, improving responsiveness, and building sustainable competitive advantage in an increasingly uncertain global environment.
TN-plastics provides medical device OEMs with the nearshoring capabilities they need: strategic manufacturing locations in Michigan and Italy, comprehensive technical expertise, FDA-registered cleanroom manufacturing, complete validation services, and a commitment to partnership excellence backed by over a century of manufacturing experience.
Whether you're developing your first nearshoring strategy or seeking to strengthen existing supply chains, TN-plastics brings the capabilities, experience, and geographic presence to support your success. Our proven track record serving the world's leading medical OEMs, production capacity from prototypes to millions of units annually, and global Tsubaki Nakashima network provide the foundation for supply chain resilience.
The question is no longer whether to pursue nearshoring, but how to implement it strategically to maximize value and minimize risk. TN-plastics is ready to be your partner in building supply chain resilience that supports innovation, quality, and competitive advantage.

